The Only Molecules That Can Pass Freely Through the Plasma Membrane Are Ones That Are
The Role of Passive Transport
Passive transport, such as diffusion and osmosis, moves materials of small molecular weight beyond membranes.
Learning Objectives
Indicate the manner in which various materials cross the prison cell membrane
Central Takeaways
Cardinal Points
- Plasma membranes are selectively permeable; if they were to lose this selectivity, the cell would no longer exist able to sustain itself.
- In passive transport, substances but move from an area of college concentration to an surface area of lower concentration, which does not require the input of energy.
- Concentration gradient, size of the particles that are diffusing, and temperature of the organization touch on the rate of diffusion.
- Some materials lengthened readily through the membrane, but others require specialized proteins, such equally channels and transporters, to conduct them into or out of the jail cell.
Fundamental Terms
- concentration gradient: A concentration slope is present when a membrane separates two different concentrations of molecules.
- passive transport: A motion of biochemicals and other atomic or molecular substances across membranes that does not require an input of chemic energy.
- permeable: Of or relating to substance, substrate, membrane or material that absorbs or allows the passage of fluids.
Introduction: Passive Transport
Plasma membranes must allow or prevent certain substances from entering or leaving a prison cell. In other words, plasma membranes are selectively permeable; they let some substances to pass through, simply not others. If they were to lose this selectivity, the cell would no longer be able to sustain itself, and it would be destroyed. Some cells crave larger amounts of specific substances than other cells; they must have a way of obtaining these materials from extracellular fluids. This may happen passively, as sure materials move back and forth, or the cell may have special mechanisms that facilitate transport. Some materials are so of import to a prison cell that it spends some of its energy (hydrolyzing adenosine triphosphate (ATP)) to obtain these materials. Red blood cells use some of their energy to do this. All cells spend the majority of their free energy to maintain an imbalance of sodium and potassium ions between the interior and exterior of the cell.
The most direct forms of membrane transport are passive. Passive ship is a naturally-occurring phenomenon and does not crave the cell to exert whatever of its free energy to attain the movement. In passive transport, substances move from an surface area of higher concentration to an area of lower concentration. A physical infinite in which there is a range of concentrations of a single substance is said to take a concentration gradient.

Passive Transport: Improvidence is a type of passive transport. Diffusion through a permeable membrane moves a substance from an surface area of loftier concentration (extracellular fluid, in this case) down its concentration gradient (into the cytoplasm).
The passive forms of transport, diffusion and osmosis, move materials of minor molecular weight across membranes. Substances diffuse from areas of high concentration to areas of lower concentration; this process continues until the substance is evenly distributed in a system. In solutions containing more one substance, each type of molecule diffuses according to its own concentration gradient, independent of the diffusion of other substances. Many factors can bear upon the rate of improvidence, including, only not limited to, concentration gradient, size of the particles that are diffusing, and temperature of the arrangement.
In living systems, diffusion of substances in and out of cells is mediated past the plasma membrane. Some materials diffuse readily through the membrane, but others are hindered; their passage is made possible past specialized proteins, such as channels and transporters. The chemistry of living things occurs in aqueous solutions; balancing the concentrations of those solutions is an ongoing problem. In living systems, diffusion of some substances would be irksome or difficult without membrane proteins that facilitate transport.
Selective Permeability
The hydrophobic and hydrophilic regions of plasma membranes aid the diffusion of some molecules and hinder the diffusion of others.
Learning Objectives
Describe how membrane permeability, concentration gradient, and molecular properties affect biological improvidence rates.
Key Takeaways
Key Points
- The interior and outside surfaces of the plasma membrane are not identical, which adds to the selective permeability of the membrane.
- Fat soluble substances are able to pass hands to the hydrophobic interior of the plasma membrane and diffuse into the cell.
- Polar molecules and charged molecules do not lengthened easily through the lipid core of the plasma membrane and must be transported beyond by proteins, sugars, or amino acids.
Key Terms
- polar: a separation of electric charge leading to a molecule or its chemical groups having an electric dipole
- amphiphilic: Having one surface consisting of hydrophilic amino acids and the opposite surface consisting of hydrophobic (or lipophilic) ones.
Selective Permeability
Plasma membranes are asymmetric: the interior of the membrane is not identical to the exterior of the membrane. In fact, at that place is a considerable departure between the array of phospholipids and proteins between the two leaflets that form a membrane. On the interior of the membrane, some proteins serve to anchor the membrane to fibers of the cytoskeleton. At that place are peripheral proteins on the exterior of the membrane that bind elements of the extracellular matrix. Carbohydrates, fastened to lipids or proteins, are also found on the outside surface of the plasma membrane. These carbohydrate complexes help the cell demark substances that the prison cell needs in the extracellular fluid. This adds considerably to the selective nature of plasma membranes.
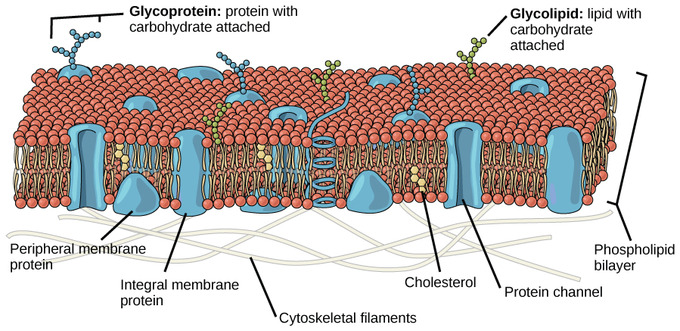
Asymmetry in Plasma Membranes: The exterior surface of the plasma membrane is non identical to the interior surface of the aforementioned membrane.
Recall that plasma membranes are amphiphilic; that is, they have hydrophilic and hydrophobic regions. This characteristic helps the movement of some materials through the membrane and hinders the motility of others. Lipid-soluble fabric with a low molecular weight tin easily sideslip through the hydrophobic lipid core of the membrane. Substances such as the fat-soluble vitamins A, D, Due east, and K readily laissez passer through the plasma membranes in the digestive tract and other tissues. Fat-soluble drugs and hormones also gain easy entry into cells and are readily transported into the body's tissues and organs. Molecules of oxygen and carbon dioxide take no charge and then pass through membranes past simple diffusion.
Polar substances nowadays problems for the membrane. While some polar molecules connect easily with the outside of a cell, they cannot readily pass through the lipid core of the plasma membrane. Additionally, while minor ions could easily sideslip through the spaces in the mosaic of the membrane, their charge prevents them from doing then. Ions such equally sodium, potassium, calcium, and chloride must take special means of penetrating plasma membranes. Simple sugars and amino acids besides need help with transport across plasma membranes, achieved past various transmembrane proteins (channels).
Diffusion
Improvidence is a process of passive transport in which molecules move from an area of higher concentration to 1 of lower concentration.
Learning Objectives
Describe diffusion and the factors that affect how materials movement beyond the cell membrane.
Key Takeaways
Key Points
- Substances diffuse according to their concentration gradient; within a system, different substances in the medium will each lengthened at dissimilar rates according to their individual gradients.
- After a substance has diffused completely through a space, removing its concentration gradient, molecules will still move effectually in the infinite, but at that place will exist no net movement of the number of molecules from one area to some other, a state known as dynamic equilibrium.
- Several factors affect the rate of improvidence of a solute including the mass of the solute, the temperature of the surround, the solvent density, and the distance traveled.
Fundamental Terms
- diffusion: The passive motion of a solute beyond a permeable membrane
- concentration gradient: A concentration gradient is present when a membrane separates two different concentrations of molecules.
Diffusion
Diffusion is a passive procedure of transport. A single substance tends to move from an area of loftier concentration to an expanse of low concentration until the concentration is equal across a space. You are familiar with diffusion of substances through the air. For case, think about someone opening a bottle of ammonia in a room filled with people. The ammonia gas is at its highest concentration in the bottle; its everyman concentration is at the edges of the room. The ammonia vapor will lengthened, or spread away, from the bottle; gradually, more and more people will odour the ammonia every bit it spreads. Materials movement within the cell 's cytosol by diffusion, and certain materials move through the plasma membrane by diffusion. Diffusion expends no energy. On the contrary, concentration gradients are a form of potential energy, dissipated as the gradient is eliminated.

Diffusion: Diffusion through a permeable membrane moves a substance from an area of high concentration (extracellular fluid, in this instance) down its concentration slope (into the cytoplasm).
Each separate substance in a medium, such every bit the extracellular fluid, has its own concentration gradient independent of the concentration gradients of other materials. In improver, each substance will diffuse according to that gradient. Inside a system, there will exist unlike rates of diffusion of the different substances in the medium.
Factors That Affect Improvidence
Molecules move constantly in a random style at a charge per unit that depends on their mass, their environment, and the amount of thermal energy they possess, which in turn is a function of temperature. This movement accounts for the diffusion of molecules through whatever medium in which they are localized. A substance will tend to move into any infinite bachelor to it until it is evenly distributed throughout it. After a substance has diffused completely through a space removing its concentration slope, molecules will still move effectually in the space, simply there will be no internet movement of the number of molecules from i area to another. This lack of a concentration gradient in which there is no net motility of a substance is known equally dynamic equilibrium. While improvidence volition become forrad in the presence of a concentration gradient of a substance, several factors touch the rate of diffusion:
- Extent of the concentration gradient: The greater the difference in concentration, the more rapid the improvidence. The closer the distribution of the material gets to equilibrium, the slower the charge per unit of diffusion becomes.
- Mass of the molecules diffusing: Heavier molecules movement more slowly; therefore, they diffuse more slowly. The reverse is truthful for lighter molecules.
- Temperature: Higher temperatures increase the energy and therefore the move of the molecules, increasing the rate of improvidence. Lower temperatures decrease the free energy of the molecules, thus decreasing the rate of diffusion.
- Solvent density: Every bit the density of a solvent increases, the rate of diffusion decreases. The molecules slow downwardly because they have a more difficult time getting through the denser medium. If the medium is less dense, diffusion increases. Because cells primarily use diffusion to motion materials within the cytoplasm, whatever increase in the cytoplasm'south density will inhibit the motion of the materials. An example of this is a person experiencing dehydration. As the body's cells lose water, the rate of diffusion decreases in the cytoplasm, and the cells' functions deteriorate. Neurons tend to be very sensitive to this issue. Dehydration frequently leads to unconsciousness and possibly coma because of the decrease in improvidence charge per unit within the cells.
- Solubility: As discussed earlier, nonpolar or lipid-soluble materials laissez passer through plasma membranes more easily than polar materials, allowing a faster charge per unit of improvidence.
- Surface surface area and thickness of the plasma membrane: Increased surface area increases the rate of diffusion, whereas a thicker membrane reduces it.
- Distance travelled: The greater the altitude that a substance must travel, the slower the rate of diffusion. This places an upper limitation on cell size. A large, spherical cell will die because nutrients or waste cannot reach or leave the center of the jail cell. Therefore, cells must either be small in size, equally in the case of many prokaryotes, or exist flattened, as with many single-celled eukaryotes.
A variation of improvidence is the process of filtration. In filtration, textile moves according to its concentration gradient through a membrane; sometimes the charge per unit of diffusion is enhanced past pressure, causing the substances to filter more than speedily. This occurs in the kidney where claret pressure forces large amounts of water and accompanying dissolved substances, or solutes, out of the blood and into the renal tubules. The rate of improvidence in this instance is nearly totally dependent on pressure level. One of the effects of high blood pressure is the appearance of protein in the urine, which is "squeezed through" by the abnormally high force per unit area.
Facilitated transport
Facilitated diffusion is a process by which molecules are transported beyond the plasma membrane with the help of membrane proteins.
Learning Objectives
Explain why and how passive transport occurs
Primal Takeaways
Key Points
- A concentration slope exists that would let ions and polar molecules to lengthened into the prison cell, just these materials are repelled past the hydrophobic parts of the prison cell membrane.
- Facilitated diffusion uses integral membrane proteins to motion polar or charged substances beyond the hydrophobic regions of the membrane.
- Aqueduct proteins can aid in the facilitated diffusion of substances by forming a hydrophilic passage through the plasma membrane through which polar and charged substances tin pass.
- Channel proteins can be open at all times, constantly allowing a particular substance into or out of the cell, depending on the concentration slope; or they can exist gated and tin can merely be opened by a particular biological signal.
- Carrier proteins assist in facilitated improvidence by bounden a detail substance, then altering their shape to bring that substance into or out of the prison cell.
Cardinal Terms
- facilitated diffusion: The spontaneous passage of molecules or ions across a biological membrane passing through specific transmembrane integral proteins.
- membrane protein: Proteins that are fastened to, or associated with the membrane of a cell or an organelle.
Facilitated transport
Facilitated ship is a type of passive ship. Dissimilar simple diffusion where materials laissez passer through a membrane without the help of proteins, in facilitated transport, likewise called facilitated diffusion, materials lengthened beyond the plasma membrane with the help of membrane proteins. A concentration slope exists that would allow these materials to lengthened into the prison cell without expending cellular energy. However, these materials are ions or polar molecules that are repelled by the hydrophobic parts of the cell membrane. Facilitated send proteins shield these materials from the repulsive force of the membrane, assuasive them to diffuse into the jail cell.
The material being transported is starting time fastened to protein or glycoprotein receptors on the exterior surface of the plasma membrane. This allows the material that is needed past the jail cell to be removed from the extracellular fluid. The substances are then passed to specific integral proteins that facilitate their passage. Some of these integral proteins are collections of beta-pleated sheets that form a channel through the phospholipid bilayer. Others are carrier proteins which demark with the substance and aid its diffusion through the membrane.
Channels
The integral proteins involved in facilitated transport are collectively referred to equally transport proteins; they function as either channels for the material or carriers. In both cases, they are transmembrane proteins. Channels are specific for the substance that is being transported. Channel proteins take hydrophilic domains exposed to the intracellular and extracellular fluids; they additionally have a hydrophilic channel through their cadre that provides a hydrated opening through the membrane layers. Passage through the channel allows polar compounds to avoid the nonpolar central layer of the plasma membrane that would otherwise slow or foreclose their entry into the prison cell. Aquaporins are aqueduct proteins that allow water to pass through the membrane at a very high rate.

Channel Proteins in Facilitated Ship: Facilitated ship moves substances down their concentration gradients. They may cantankerous the plasma membrane with the help of channel proteins.
Aqueduct proteins are either open at all times or they are "gated," which controls the opening of the channel. The zipper of a particular ion to the channel protein may control the opening or other mechanisms or substances may be involved. In some tissues, sodium and chloride ions laissez passer freely through open up channels, whereas in other tissues, a gate must exist opened to allow passage. An case of this occurs in the kidney, where both forms of channels are found in dissimilar parts of the renal tubules. Cells involved in the manual of electric impulses, such as nervus and musculus cells, accept gated channels for sodium, potassium, and calcium in their membranes. Opening and closing of these channels changes the relative concentrations on opposing sides of the membrane of these ions, resulting in the facilitation of electric transmission along membranes (in the case of nerve cells) or in muscle contraction (in the case of muscle cells).
Carrier Proteins
Another type of poly peptide embedded in the plasma membrane is a carrier protein. This protein binds a substance and, in doing and then, triggers a modify of its own shape, moving the bound molecule from the exterior of the prison cell to its interior; depending on the slope, the material may motility in the reverse management. Carrier proteins are typically specific for a single substance. This adds to the overall selectivity of the plasma membrane. The exact mechanism for the change of shape is poorly understood. Proteins can alter shape when their hydrogen bonds are affected, simply this may non fully explain this machinery. Each carrier protein is specific to one substance, and at that place are a finite number of these proteins in any membrane. This can cause problems in transporting plenty of the textile for the cell to function properly.

Carrier Proteins: Some substances are able to move downwardly their concentration gradient across the plasma membrane with the assist of carrier proteins. Carrier proteins change shape as they move molecules across the membrane.
An instance of this procedure occurs in the kidney. Glucose, water, salts, ions, and amino acids needed by the body are filtered in 1 part of the kidney. This filtrate, which includes glucose, is and then reabsorbed in another part of the kidney. Because in that location are only a finite number of carrier proteins for glucose, if more glucose is present than the proteins can handle, the excess is not transported; information technology is excreted from the trunk in the urine. In a diabetic individual, this is described as "spilling glucose into the urine." A dissimilar group of carrier proteins called glucose send proteins, or GLUTs, are involved in transporting glucose and other hexose sugars through plasma membranes within the trunk.
Channel and carrier proteins ship material at unlike rates. Aqueduct proteins ship much more quickly than practise carrier proteins. Channel proteins facilitate diffusion at a rate of tens of millions of molecules per 2nd, whereas carrier proteins work at a rate of a grand to a million molecules per second.
Osmosis
Osmosis is the movement of water across a membrane from an expanse of low solute concentration to an area of high solute concentration.
Learning Objectives
Depict the process of osmosis and explain how concentration gradient affects osmosis
Key Takeaways
Fundamental Points
- Osmosis occurs according to the concentration gradient of water beyond the membrane, which is inversely proportional to the concentration of solutes.
- Osmosis occurs until the concentration gradient of water goes to aught or until the hydrostatic force per unit area of the water balances the osmotic pressure.
- Osmosis occurs when in that location is a concentration slope of a solute within a solution, only the membrane does not allow diffusion of the solute.
Key Terms
- solute: Any substance that is dissolved in a liquid solvent to create a solution
- osmosis: The internet move of solvent molecules from a region of high solvent potential to a region of lower solvent potential through a partially permeable membrane
- semipermeable membrane: A blazon of biological membrane that will allow sure molecules or ions to pass through it by improvidence and occasionally by specialized facilitated improvidence
Osmosis and Semipermeable Membranes
Osmosis is the motion of water through a semipermeable membrane according to the concentration gradient of h2o across the membrane, which is inversely proportional to the concentration of solutes. Semipermeable membranes, too termed selectively permeable membranes or partially permeable membranes, permit certain molecules or ions to pass through by diffusion.
While diffusion transports materials beyond membranes and within cells, osmosis transports just water beyond a membrane. The semipermeable membrane limits the diffusion of solutes in the water. Not surprisingly, the aquaporin proteins that facilitate h2o motility play a large role in osmosis, most prominently in red blood cells and the membranes of kidney tubules.
Mechanism of Osmosis
Osmosis is a special case of diffusion. Water, like other substances, moves from an area of high concentration to one of depression concentration. An obvious question is what makes water motility at all? Imagine a beaker with a semipermeable membrane separating the ii sides or halves. On both sides of the membrane the water level is the same, but there are different concentrations of a dissolved substance, or solute, that cannot cross the membrane (otherwise the concentrations on each side would be balanced by the solute crossing the membrane). If the volume of the solution on both sides of the membrane is the aforementioned merely the concentrations of solute are different, and so there are different amounts of water, the solvent, on either side of the membrane. If there is more than solute in one expanse, then there is less water; if there is less solute in i area, then in that location must be more water.
To illustrate this, imagine two total glasses of water. One has a unmarried teaspoon of sugar in it, whereas the second one contains one-quarter cup of sugar. If the total book of the solutions in both cups is the same, which cup contains more than water? Because the large amount of saccharide in the second cup takes up much more infinite than the teaspoon of sugar in the commencement cup, the first cup has more water in information technology.

Osmosis: In osmosis, h2o always moves from an area of higher h2o concentration to one of lower concentration. In the diagram shown, the solute cannot pass through the selectively permeable membrane, but the h2o can.
Returning to the beaker example, recall that it has a mixture of solutes on either side of the membrane. A principle of diffusion is that the molecules move around and will spread evenly throughout the medium if they can. However, but the textile capable of passing through the membrane will diffuse through it. In this example, the solute cannot lengthened through the membrane, but the water can. Water has a concentration gradient in this system. Thus, h2o will diffuse down its concentration gradient, crossing the membrane to the side where it is less concentrated. This diffusion of water through the membrane—osmosis—will continue until the concentration gradient of water goes to zero or until the hydrostatic pressure of the h2o balances the osmotic pressure level. In the beaker example, this means that the level of fluid in the side with a higher solute concentration will go up.
Tonicity
Tonicity, which is direct related to the osmolarity of a solution, affects osmosis past determining the direction of water flow.
Learning Objectives
Ascertain tonicity and depict its relevance to osmosis
Fundamental Takeaways
Key Points
- Osmolarity describes the total solute concentration of a solution; solutions with a low solute concentration have a low osmolarity, while those with a high osmolarity take a high solute concentration.
- Water moves from the side of the membrane with lower osmolarity (and more than water) to the side with higher osmolarity (and less water).
- In a hypotonic solution, the extracellular fluid has a lower osmolarity than the fluid within the prison cell; water enters the jail cell.
- In a hypertonic solution, the extracellular fluid has a higher osmolarity than the fluid within the prison cell; water leaves the cell.
- In an isotonic solution, the extracellular fluid has the same osmolarity as the cell; in that location volition be no cyberspace movement of water into or out of the cell.
Key Terms
- osmolarity: The osmotic concentration of a solution, normally expressed as osmoles of solute per litre of solution.
- hypotonic: Having a lower osmotic pressure than another; a cell in this environment causes h2o to enter the prison cell, causing information technology to nifty.
- hypertonic: having a greater osmotic pressure than another
- isotonic: having the aforementioned osmotic force per unit area
Tonicity
Tonicity describes how an extracellular solution can change the volume of a cell by affecting osmosis. A solution's tonicity frequently direct correlates with the osmolarity of the solution. Osmolarity describes the total solute concentration of the solution. A solution with low osmolarity has a greater number of water molecules relative to the number of solute particles; a solution with high osmolarity has fewer water molecules with respect to solute particles. In a situation in which solutions of ii different osmolarities are separated by a membrane permeable to water, though not to the solute, h2o volition move from the side of the membrane with lower osmolarity (and more h2o) to the side with higher osmolarity (and less h2o). This effect makes sense if you remember that the solute cannot motility across the membrane, and thus the only component in the system that can move—the water—moves along its own concentration slope. An important distinction that concerns living systems is that osmolarity measures the number of particles (which may be molecules) in a solution. Therefore, a solution that is cloudy with cells may take a lower osmolarity than a solution that is clear if the second solution contains more than dissolved molecules than in that location are cells.
Hypotonic Solutions
Iii terms—hypotonic, isotonic, and hypertonic—are used to relate the osmolarity of a jail cell to the osmolarity of the extracellular fluid that contains the cells. In a hypotonic situation, the extracellular fluid has lower osmolarity than the fluid inside the cell, and water enters the cell. (In living systems, the point of reference is always the cytoplasm, and then the prefix hypo- ways that the extracellular fluid has a lower concentration of solutes, or a lower osmolarity, than the cell cytoplasm. ) It also means that the extracellular fluid has a higher concentration of water in the solution than does the cell. In this situation, water will follow its concentration slope and enter the cell, causing the cell to expand.

Changes in Jail cell Shape Due to Dissolved Solutes: Osmotic force per unit area changes the shape of cerise claret cells in hypertonic, isotonic, and hypotonic solutions.
Hypertonic Solutions
As for a hypertonic solution, the prefix hyper- refers to the extracellular fluid having a higher osmolarity than the cell'due south cytoplasm; therefore, the fluid contains less water than the cell does. Because the prison cell has a relatively higher concentration of h2o, h2o will exit the cell, and the prison cell will shrink.
Isotonic Solutions
In an isotonic solution, the extracellular fluid has the same osmolarity as the cell. If the osmolarity of the cell matches that of the extracellular fluid, there will be no net movement of water into or out of the cell, although h2o volition still move in and out.
Blood cells and plant cells in hypertonic, isotonic, and hypotonic solutions have on feature appearances. Cells in an isotonic solution retain their shape. Cells in a hypotonic solution smashing as water enters the prison cell, and may burst if the concentration gradient is large enough between the within and outside of the cell. Cells in a hypertonic solution compress equally water exits the cell, becoming shriveled.
Osmoregulation
Osmoregulation is the process by which living things regulate the furnishings of osmosis in order to protect cellular integrity.
Learning Objectives
Describe the methods by which organisms regulate the effects of osmosis on their cells
Cardinal Takeaways
Cardinal Points
- In a hypotonic environment, h2o enters a cell, causing information technology to neat and outburst (lyse).
- In a hypertonic environment, water leaves a cell, causing information technology to shrink and dice.
- In plants, in a hypotonic environment, the inflow of water produces turgor pressure level, which stiffens the prison cell walls of the plant, while in a hypertonic surround, turgor force per unit area is lost, and the plant wilts.
- Some protists that lack cell walls have contractile vacuoles, which collect excess h2o from the cell and pump it out, keeping the cell from lysing.
- Freshwater fish living in a hypotonic environment accept in salt through their gills and excrete diluted urine to rid themselves of excess h2o; saltwater fish living in a hypertonic environment secrete salt through their gills and excrete highly concentrated urine.
- In vertebrates, osmoreceptors monitor the concentration of solutes in the claret, and albumin controls osmotic pressure in tissues.
Key Terms
- osmoregulation: the homeostatic regulation of osmotic pressure in the body in order to maintain a constant water content
- hypertonic: having a greater osmotic pressure than some other
- hypotonic: Having a lower osmotic pressure than another; a jail cell in this environment causes water to enter the cell, causing it to great.
Tonicity in Living Systems
Tonicity is the ability of a solution to exert an osmotic pressure level upon a membrane. There are three types of tonicity: hypotonic, hypertonic, and isotonic. In a hypotonic environment, h2o enters a jail cell, and the prison cell swells. In a hypertonic solution, water leaves a jail cell and the jail cell shrinks. In an isotonic condition, the relative concentrations of solute and solvent are equal on both sides of the membrane. At that place is no internet water move; therefore, there is no alter in the size of the prison cell. If either the hypo- or hyper- condition goes to backlog, the cell's functions become compromised, and the cell may exist destroyed.
A red blood jail cell will outburst, or lyse, when it swells beyond the plasma membrane 's capability to expand. The membrane resembles a mosaic with discrete spaces between the molecules comprising it. If the cell swells and the spaces betwixt the lipids and proteins become likewise large, the cell volition break apart.
In contrast, when excessive amounts of h2o go out a red blood cell, the cell shrinks, or crenates. This has the event of concentrating the solutes left in the prison cell, making the cytosol denser and interfering with diffusion within the prison cell. The prison cell's ability to function will exist compromised and may also result in the death of the cell.

Turgor Pressure and Tonicity in a Plant Cell: The turgor pressure level within a plant cell depends on the tonicity of the solution in which it is bathed.
Osmoregulation
Various living things take ways of controlling the effects of osmosis —a mechanism chosen osmoregulation. Some organisms, such as plants, fungi, bacteria, and some protists, have cell walls that surround the plasma membrane and prevent cell lysis in a hypotonic solution. The plasma membrane can simply expand to the limit of the cell wall, and then the prison cell will not lyse. In fact, in plants, the cellular environment is always slightly hypotonic to the cytoplasm, and h2o will always enter a cell if water is bachelor. This inflow of water produces turgor pressure level, which stiffens the cell walls of the constitute. In nonwoody plants, turgor pressure supports the establish. Conversely, if the plant is non watered, the extracellular fluid will get hypertonic, causing water to leave the jail cell. In this condition, the cell does not compress because the prison cell wall is non flexible. Notwithstanding, the prison cell membrane detaches from the wall and constricts the cytoplasm. This is called plasmolysis. Plants lose turgor pressure in this status and wilt.

Turgor Pressure level and Plasmolysis: Without adequate water, the plant on the left has lost turgor pressure, visible in its wilting; the turgor pressure is restored past watering information technology (right).
Tonicity is a concern for all living things. For case, paramecia and amoebas, which are protists that lack cell walls, have contractile vacuoles. This vesicle collects excess water from the jail cell and pumps it out, keeping the cell from lysing every bit it takes on water from its environment.

Contractile Vacuoles: A paramecium's contractile vacuole, here visualized using bright field light microscopy at 480x magnification, continuously pumps h2o out of the organism'southward body to keep information technology from bursting in a hypotonic medium.
Many marine invertebrates accept internal common salt levels matched to their environments, making them isotonic with the water in which they alive. Fish, withal, must spend approximately five percent of their metabolic energy maintaining osmotic homeostasis. Freshwater fish live in an environment that is hypotonic to their cells. These fish actively take in salt through their gills and excrete diluted urine to rid themselves of backlog water. Saltwater fish live in the reverse environs, which is hypertonic to their cells, and they secrete salt through their gills and excrete highly concentrated urine.
In vertebrates, the kidneys regulate the corporeality of water in the body. Osmoreceptors are specialized cells in the encephalon that monitor the concentration of solutes in the blood. If the levels of solutes increase beyond a certain range, a hormone is released that retards water loss through the kidney and dilutes the blood to safer levels. Animals also have high concentrations of albumin (produced by the liver) in their blood. This protein is also large to pass easily through plasma membranes and is a major factor in controlling the osmotic pressures applied to tissues.
Source: https://courses.lumenlearning.com/boundless-biology/chapter/passive-transport/
0 Response to "The Only Molecules That Can Pass Freely Through the Plasma Membrane Are Ones That Are"
Post a Comment